Notice to Industry – Updates to the federal management of chronic wasting disease in farmed cervids
December 12, 2017 – Ottawa, ON – The Canadian Food Inspection Agency (CFIA) is updating its national chronic wasting disease (CWD) program to better focus on disease prevention and risk management. A three-month extension to the implementation date has been granted to give producers, regional administrators and status assessors, provinces and territories more time to prepare for the upcoming change.
Disease management is a shared responsibility. Efforts to eradicate CWD in the farmed cervid population have not been successful. As a result, the CFIA's new approach aims to reduce the risk of the disease spreading by encouraging producers to adopt strong risk mitigation measures.
Enrolling in a Voluntary Herd Certification Program (VHCP) is important to help prevent the introduction of CWD to a farm. A VHCP requires enrolled producers to take measures to mitigate the risk of CWD, including ongoing surveillance testing of mature dead cervids and implementation of biosecurity measures.
Starting April 1, 2018, the CFIA will only respond to and compensate producers enrolled in a VHCP. A transition period is being provided throughout 2018 to give producers time to enroll in and complete 12 months in a VHCP.
Starting April 1, 2019, the CFIA will only respond to and compensate affected producers who have been enrolled in and compliant with a VHCP for at least 12 months.
CFIA's response includes movement controls, ordering destruction and disposal of infected herds, and providing compensation to producers.
More information about VHCPs is available in the Accredited Veterinarian's Manual, chapter 13.
Key dates:
June 30, 2017: Announcement of program change to producers, associations and other affected organizations.
April 1, 2018: New program starts. As of this date, producers need to be enrolled in a VHCP to be eligible for CFIA response and compensation.
April 1, 2018 to March 31, 2019: During this transition period, producers should contact the regional administrator of the VHCP available in their area and consider enrolling in the program in order to control the risk of CWD entering their herd and to be eligible for federal response and compensation.
April 1, 2019: Full implementation of program change. As of this date, only producers that have been enrolled in and compliant with a VHCP for at least 12 months will be eligible for the CFIA's full response and compensation.
Chapter 20
Agriculture—Mitigating Risk of Livestock Diseases
1.0 MAIN POINTS
The Ministry of Agriculture had effective processes, for the 12-month period ending August 2017, to minimize the risk of occurrence and spread of diseases of farmed animals in Saskatchewan other than in the two areas noted below.
To prevent and control the spread of diseases among farmed animals in Saskatchewan, the Ministry does the following. It maintains a list of 14 diseases it considers of sufficient threat to require provincial notification and monitoring. It carries out surveillance activities on almost one-half of its provincial notifiable diseases.
In the event of positive cases of notifiable diseases, for three notifiable diseases, the Ministry has formal written plans to respond. For the remaining notifiable diseases, it monitors the sufficiency of actions taken to treat diseased animals, and actions taken to prevent further spread of the disease.
Furthermore, the Ministry identifies new or emerging diseases, and has a documented plan to prevent foreign animal diseases from entering the province. It gives producers adequate information to educate them on disease risks, and disease prevention and control techniques.
However, the Ministry did not maintain support for its decisions on which diseases to include on the list, which diseases warrant surveillance, or the extent of its involvement in reported cases of livestock diseases.
While the Ministry maintains records on positive cases of notifiable livestock diseases to summarize key information about the case, its records are not complete. These records are the Ministry’s main monitoring tool to determine if veterinarians and those contracted by the Ministry took sufficient actions to limit the spread of disease.
The livestock sector in Saskatchewan had average annual revenues around $2 billion for the last three years. Disease outbreaks can impose significant effects on production, price and value of livestock products.
2.0 INTRODUCTION
snip...see full text;
Alberta
The total number of CWD cases detected in wild deer in Alberta since September 2005 is 592.
Chronic Wasting Disease (CWD) Surveillance Update: September 27, 2017
In conjunction with the new hunting seasons, the 2017/18 CWD surveillance program is again underway. We began hunter surveillance in 1998 and have one of the best continuous datasets documenting the occurrence and patterns of CWD in wild cervids, and specifically in prairie/parkland ecosystems. The continued support of hunters and landowners over the previous 19 years is the basis for the strength in the surveillance data.
For 2017/18 the basic program remains much the same as in recent years. The mandatory area for submitting deer heads expanded to include (WMUs 206, 208, 228, 240, 242) in association with an infected deer detected in WMU 242 in 2015/16. In addition, we added new WMUs of Special Concern (250, 252, 260) in response to a case identified in WMU 250 late last year. Additional deer heads from the units of Special Concern will provide perspective for the outlier case and these WMUs will be included in the mandatory area next fall. We also expanded the number of 24hr freezers, including freezers at Brooks, Morinville, and Smoky Lake. And the freezer in Drumheller was moved to a new location. Visit our web page below for a link to a map of all of the WMUs that are Mandatory or Special Concern, as well as the complete list, addresses, and a map showing locations of all the freezers available during the rifle seasons.
Note that the head drop-off freezers are ONLY available from mid October to mid December. However, any time throughout the year, heads can be submitted at any Fish and Wildlife office during their office hours. See page 13 of the 2017 Alberta Guide to Hunting Regulations for office locations and phone numbers.
Please also note that for biosafety and logistical reasons, we are unable to return heads to a hunter. If you wish to keep any part of the head or antlers, you should remove it before submitting the remaining portion for CWD testing. Additional information is provided at the web page below.
Additional information about preparing and submitting heads and specific freezer locations can be found at:
The success of the CWD surveillance program relies heavily on participation by hunters, guides, and landowners to ensure a successful harvest that provides heads to be tested. We gratefully acknowledge the efforts of one and all.
The total number of CWD cases detected in wild deer in Alberta since September 2005 is 592.
Note that hunters receive NEGATIVE test results directly at the email address associated with their individual AlbertaRELM account. The email process is the only notification hunters receive when their animal is NEGATIVE for CWD.
As in the past, hunters who harvest a CWD POSITIVE deer are contacted directly by phone (see below).
Patterns of CWD in Alberta
There are significant overall patterns of disease occurrence in Alberta. CWD continues to occur primarily in mule deer in comparison to white-tailed deer despite testing large numbers of both species. Similarly males are more likely to be infected than females.
Analyses of previous data determined the weighted CWD occurrence in Alberta is:
- Mule Deer: male 1.00 female 0.4
- White-tailed Deer: male 0.3 female 0.1
Thus male mule deer are the most likely, and female white-tailed deer the least likely to be infected with CWD.
The geographic distribution of CWD is clustered in some WMUs but continues to expand westward.
The finding of CWD in a moose near the South Saskatchewan River valley in 2012 is the first such case identified in Canada.
Specific information about the CWD hunter surveillance program is provided at:
The CWD Freezer Locations currently posted on the Information for Hunters page has all the correct information for 2016. Note that the freezers generally are available each year only between mid-October and late December. Current information also is available from any Fish and Wildlife office.
- CWD surveillance is focused on the Alberta/Saskatchewan border; however, hunter-killed deer (and elk) are accepted from anywhere in the province (as in all previous years)
- Ongoing NEGATIVE test results are made available to individual hunters; when test results are available, the hunter receives an email that provides the negative result
- Ongoing POSITIVE test results are provided by phone directly to the hunter who harvested the infected deer
2016 Fall CWD Surveillance Results
In 2016/17 we tested a total of 5112 heads and detected CWD in 179 animals (3.5%; up from 2.4% in 2015/16). The positives included 178 deer (154 mule deer, 23 white-tail, 1 unknown deer; 136 males, 41 females, 1 unknown gender) and 1 male elk. As in previous years the majority of cases were mule deer (154 of 179; 86%), particularly mule deer bucks (119 of 179; 66%).
Also as in previous years, species- and gender-specific differences are apparent. In the 4944 heads that were suitable for determining disease status, CWD was detected in:
- 5.4% of 2833 mule deer
- 1.5% of 1494 white-tailed deer
- 0.2% of 431 elk (primarily from CFB Suffield)
- 0 of 176 moose (primarily from CFB Wainwright)
In the 4312 deer for which gender/sex was reported, CWD was detected in:
- 8.1% of 1473 male mule deer
- 2.6% of 1349 female mule deer
- 1.7% of 1071 male whitetails
- 1.3% of 473 female whitetails
The disease continues to expand further westward into central Alberta. In the 2016/17 surveillance sample, CWD was again detected beyond the known range in the province (further up the Red Deer River in Wildlife Management Unit (WMU) 158, in WMU 230 in the Battle River watershed, and in WMU 254 in the Vermilion River watershed). These units are adjacent to previous cases and indicate further geographic spread of CWD along major waterways. However, the finding of CWD in a white-tailed deer in WMU 250 northeast of Fort Saskatchewan is a significant westward extension of the known occurrence in the North Saskatchewan River watershed.
We also detected CWD in a bull elk from WMU 732 (Canadian Forces Base Suffield). Since 2012, we tested 1973 elk from WMU 732 and this is the first one found to have CWD (0.05%). However, the disease is well established in mule deer and white-tailed deer in areas outside the military base along the Red Deer and South Saskatchewan rivers.
Hunters continue to support the program and are providing a solid foundation on which we can monitor CWD as it spreads among eastcentral deer populations.
To learn more about CWD Surveillance in Alberta, see:
For past CWD surveillance results and a general timeline of CWD in Alberta, see:
Attention Hunters!
Submit deer heads for CWD testing at any Fish and Wildlife office during their office hours or any of the forty-nine 24-hour freezers in Edmonton, Calgary, and across eastern Alberta during rifle seasons.
Submission of deer heads for CWD testing is MANDATORY in eastern Alberta from Cold Lake south to the US border.
For more details, see:
CWD Map and Statistics
News Releases and Information Bulletins
- Nineteen new cases of chronic wasting disease in wild deer- Apr 1, 2011
- New cases of chronic wasting disease found in wild deer- Mar 19, 2010
- Eight new cases of chronic wasting disease detected in wild deer- Mar 20, 2009
- Opportunities abound for Alberta hunters in 2007 guide/Alberta continues program to manage chronic wasting disease- Jul 16, 2007
- Testing completed for chronic wasting disease winter program- May 8, 2007
- Expanded chronic wasting disease testing discovers three more cases- Apr 5, 2007
- Alberta takes action on chronic wasting disease in wild deer- Feb 22, 2007
- Three more cases of chronic wasting disease found in wild deer- Dec 21, 2006
- Alberta hunters asked to assist with CWD control efforts- Sep 15, 2006
- Winter efforts to control CWD in wild deer in Alberta wrap up- Apr 18, 2006
- Winter tests find one more case of CWD in wild deer in Alberta- Mar 3, 2006
- Four more cases of CWD found in wild deer in Alberta- Feb 17, 2006
- First case of CWD found in wild deer killed by hunter in Alberta- Dec 9, 2005
- No chronic wasting disease found in latest culled deer- Nov 4, 2005
- Chronic wasting disease found in two more wild deer in Alberta- Oct 3, 2005
- Chronic wasting disease found in a wild deer in Alberta- Sep 2, 2005
Page Information
Updated: Oct 4, 2017
British Columbia
CWD has not been identified in any BC sample to date, but continued surveillance is required to have confidence in our CWD free status.
CHRONIC WASTING DISEASE
Information for Hunters
The B.C. Chronic Wasting Disease (CWD) Program is led by the Wildlife Health Program and supported by a wide group of agencies and BC residents who are concerned about this important disease. Surveillance continues in response to new positive cases in deer, elk and moose in Alberta. The Program focuses on prevention, surveillance and outreach to ensure that CWD does not become a problem for deer species in B.C. 2016 Results are in: NO POSITIVES! Since 2002 we have tested 3442 cervid (deer species) heads for CWD. These heads include hunter harvests, road killed and clinical suspect animals. CWD has not been identified in any BC sample to date, but continued surveillance is required to have confidence in our CWD free status. Be Aware of the Risks If you hunt in areas where CWD affects wild deer, do not bring high risk tissues back to B.C. Infected animals or tissues can contaminate soil and infect BC wildlife.
Number of samples (heads) submitted by harvest year and Region
snip...see;
Manitoba
CWD has not been detected in Manitoba.
Chronic Wasting Disease
Chronic wasting disease (CWD) is a fatal disease of the central nervous system of deer and elk. This disease belongs to a group of diseases called transmissible spongiform encephalopathies (TSEs). TSEs tend to be species specific and most are not believed by scientists to be naturally transmissible between different species.
CWD has not been detected in Manitoba. To date, more than 2,300 deer and 1,400 elk have been tested and all have tested negative.
Besides CWD, other animal TSEs include:
- scrapie of domestic sheep;
- bovine spongiform encephalopathy (BSE) or mad cow disease in cattle; and
- Creutzfeldt-Jakob Disease (CJD), a human disease found worldwide.
CWD is caused by an accumulation of abnormal proteins called prions, which causes degeneration of the brain cells. As the cells die, holes are created in the tissue, giving the brain a spongy-like appearance under a microscope. Once the disease has progressed, brain function is impaired with resulting changes in the animal’s behaviour.
Infected deer and elk show abnormal behaviour accompanied by progressive weight loss. In later stages of the disease, affected animals show signs of extreme weight loss, repetitive behaviour, drowsiness, lack of coordination, drooping head and ears, drooling, and increased drinking and urination.
Risks
There is no known case of a human being infected with CWD. Research suggests that this disease is not naturally transmissible to humans, pets or other domestic livestock.
The exact method of transmission has not been identified. Evidence suggests that the disease can pass from animal to animal by direct contact or through contamination of feed, soil, and water sources with saliva, urine and/or faeces from infected animals.
Currently, there is no approved live animal test for CWD. Diagnosis is made by microscopic examination of the brain and other tissues from dead animals.
Although progress has been made in understanding the disease, much is still unknown and active research is continuing. It is best to be cautious concerning which species can get CWD until there is conclusive information.
History
CWD
has not been found in Manitoba, however, it has been detected in many areas of
North America and in Korea. CWD was first identified in the late 1960’s in captive deer research herds in Colorado and Wyoming. In the early 1980’s, it was detected in free-ranging deer and elk in northeastern Colorado and southeastern Wyoming. It has since been found in farmed deer or elk herds in Saskatchewan, Alberta, Colorado, Wyoming, Montana, Kansas, Nebraska, South Dakota, New York, Minnesota, Oklahoma, Wisconsin, and Korea. CWD has also been detected in free-ranging deer or elk in Colorado, Wyoming, Illinois, Utah, New Mexico, Wisconsin, South Dakota, New York, Nebraska and Saskatchewan.
Consumption
The World Health Organization (WHO) has stated that, based on its review of the science, there is currently no evidence to indicate that CWD can affect humans. However, the WHO recommends that all products from animals known to be infected with any prion disease should not be consumed.
In Manitoba, harvested deer and elk are safe to consume. There is no evidence of CWD in Manitoba, the disease has not been detected in farmed elk, or wild deer or elk. Over 1,400 elk and 2,300 deer from the Riding Mountain and Duck Mountain areas and the southern part of the province have been tested for CWD - all tested negative.
Precautions
It is recommended that hunters follow the general precautions when field dressing an animal:
- wear rubber gloves when field dressing carcasses;
- minimize handling brain, eye, or spinal tissues;
- https://www.ontario.ca/page/chronic-wasting-disease
- debone the carcass, and avoid cutting through the spine;
- thoroughly clean knives with soap and warm water; and
- wash hands with soap and warm water.
If you observe an animal that appears to be sick, do not shoot the animal. Note the precise location and contact the nearest
Manitoba Conservation and Water Stewardship office. Manitoba Conservation will collect and test any wild deer or elk that appears sick and is exhibiting signs of being infected with CWD. If you kill a deer or elk that is unhealthy and extremely thin
do not field dress the animal. Attach the game tag and contact the nearest
Manitoba Conservation and Water Stewardship office. Hunters who surrender a diseased or abnormal animal will be eligible for a replacement licence at no charge.
Manitoba Conservation is working cooperatively with other jurisdictions regarding the prevention, containment and eradication
initiatives of chronic wasting disease.
Ontario
There’s no evidence the disease is in Ontario, but it’s important to be vigilant. It would harm wildlife and the economy.
Testing for 2016 is complete; all test results were negative for CWD.
2017 Chronic wasting disease testing
The Ministry of Natural Resources and Forestry conducts an annual surveillance program for chronic wasting disease testing in deer. This year, scientists will be testing deer harvested in Wildlife Management Units 64A, 64B, 65, 66A, 66B, 67, 69A-2, 69A-3, and 69B.
If you harvest a deer in these Wildlife Management Units you can participate in the testing program by:
- giving roving crews a small tissue sample from the head of your deer
- dropping off your deer's head at a Ministry of Natural Resources and Forestry freezer within a few days of harvest.
2017 Chronic wasting disease freezer depot locations
snip...see locations;
Quebec
Currently, the Department has no indication that the CWD can be present in Quebec.
Quebec deer under observation
It is essential to quickly detect the introduction of CWD in Quebec in order to increase the possibilities of eliminating the disease and limit its spread, while reducing the costs associated with these interventions.
Since 2007, the Department, in collaboration with the Quebec Ministry of Agriculture, Fisheries and Food, has been conducting a wild deer monitoring program in the sectors most at risk for the introduction of CWD. , in the extreme south of Quebec. In the past 10 years, more than 8,200 deer have been analyzed, and no cases of CWD have been detected.
 |
Number of white-tailed deer analyzed for chronic debilitating disease (CWD) in southern Quebec from October 2007 to December 2016.
|
Originally, the program was primarily based on the collection and analysis of white-tailed deer in a road accident, but since 2013, the vast majority of animals analyzed for CWD have been deer killed at the time of the accident. hunting, particularly in the Estrie and Montérégie regions.
In the rest of the province, the risk of introduction is considered lower. As a result, surveillance is less intensive. The specimens analyzed are mainly cervids showing clinical signs that may be associated with CWD. Although small in number, they are a priority for sampling since this category of specimens is more likely to be infected with CWD.
- Wildlife Disease Surveillance 2011-2014, Quebec Strategy on Wildlife Health. Chronic debilitating disease of cervids, page 29 to 41. ( PDF Format, 4.45 MB )
Saskatchewan
CWD TO DATE TOTAL 50 CASES FOR 2017
Chronic Wasting Disease Testing in Saskatchewan
| | 2017 Results |
|---|
| Species | Negative | Positive | Total |
|---|
| Elk | 7 | 0 | 7 |
| Moose | 10 | 0 | 10 |
| Mule Deer | 8 | 5 | 13 |
| White-Tailed Deer | 18 | 2 | 20 |
| Total | 43 | 7 | 50 |
*Includes both hunter surveillance and diagnostic samples
SEE Saskatchewan CWD MAP
SEE Saskatchewan previous CWD year totals;
| Year | Mule Deer | White-Tailed Deer | Elk | Moose | Total |
|---|
| Pos | Neg | Pos | Neg | Pos | Neg | Pos | Neg | Pos | Neg |
|---|
| 19971 | 0 | 3 | 0 | 36 | 0 | 0 | 0 | 0 | 0 | 39 |
| 19981 | 0 | 91 | 0 | 18 | 0 | 2 | 0 | 0 | 0 | 111 |
| 19991 | 0 | 79 | 0 | 57 | 0 | 44 | 0 | 0 | 0 | 180 |
| 20001 | 1 | 184 | 0 | 726 | 0 | 89 | 0 | 0 | 1 | 999 |
| 2001 Spring1 | 1 | 154 | 0 | 58 | 0 | 0 | 0 | 0 | 1 | 212 |
| 2001 Fall | 0 | 1077 | 0 | 2236 | 0 | 340 | 0 | 0 | 0 | 3653 |
| 2002 Spring | 1 | 134 | 0 | 24 | 0 | 0 | 0 | 0 | 1 | 158 |
| 2002 Fall | 7 | 3244 | 2 | 2413 | 0 | 163 | 0 | 0 | 9 | 5820 |
| 2003 Fall | 22 | 2830 | 0 | 1922 | 0 | 153 | 0 | 0 | 22 | 4905 |
| 2004 Fall | 30 | 5265 | 2 | 1439 | 0 | 9 | 0 | 0 | 32 | 6713 |
| 2005 Fall2 | 25 | 2635 | 10 | 938 | 0 | 47 | 0 | 4 | 35 | 3624 |
| 2006 Fall2 | 27 | 2343 | 22 | 1497 | 0 | 164 | 0 | 10 | 49 | 4014 |
| 2007 Fall2 | 32 | 2561 | 13 | 1729 | 2 | 90 | 0 | 4 | 47 | 4384 |
| 2008 Fall2 | 43 | 3668 | 5 | 828 | 1 | 214 | 0 | 27 | 49 | 4737 |
| 2009 Fall2 | 34 | 2219 | 3 | 378 | 0 | 71 | 0 | 20 | 37 | 2688 |
| 2010 Fall2 | 31 | 792 | 4 | 552 | 0 | 51 | 0 | 18 | 35 | 1413 |
| 2011 Fall2 | 27 | 401 | 5 | 419 | 1 | 52 | 0 | 19 | 33 | 891 |
| 2012 Fall2 | 26 | 247 | 7 | 209 | 3 | 36 | 0 | 14 | 36 | 506 |
| 20133 | 13 | 4 | 0 | 21 | 0 | 2 | 0 | 8 | 13 | 35 |
| 20143 | 13 | 17 | 6 | 17 | 2 | 4 | 0 | 7 | 21 | 45 |
| 2015 | 21 | 87 | 5 | 95 | 0 | 15 | 1 | 24 | 27 | 221 |
| 2016 | 33 | 140 | 14 | 162 | 2 | 20 | 0 | 25 | 49 | 347 |
| TOTAL | 387 | 28175 | 98 | 15774 | 11 | 1566 | 1 | 180 | 497 | 45695 |
Notes:
This table does not include those specimens which were deemed unsuitable for testing.
1From 1997 until the fall of 2000, only the brain section (obex) was used to diagnosis CWD. Starting in 2001, tonsils and/or retropharyngeal lymph nodes were also examined.
2Starting in the fall of 2005 animals under 1 year of age were no longer tested under the program because detectable infection is rare in a young animal and therefore not cost effective in terms of surveillance.
3The hunter surveillance program was discontinued in 2012 and the 2013 and 2014 numbers are from diagnostic samples.
Yukon
Testing from 2001-2017 shows that the Yukon has disease free herds.
The Yukon Agriculture branch works with the federal government, other Yukon government departments and industry to monitor animal and plant diseases.
CWD is a progressive, fatal, degenerative disease of the brain affecting cervids (elk, mule deer, reindeer and white-tailed deer). It belongs to a group of related diseases called Transmissible Spongiform Encephalopathies (TSE's), which include Scrapie in sheep and goats, Bovine Spongiform Encephalopathy (BSE) in cattle and Creutzfeldt-Jakob Disease (CJD) in humans. CWD is not the same as BSE.
TSE's are caused by abnormal proteins, called prions, which accumulate in the brain. There is currently no treatment or vaccine available. In order to determine if an animal is CWD free, testing must be done post-mortem. Learn more about preventing CWD in this
fact sheet 
544 KB.
(*Note that the national standards for CWD Voluntary Herd Certification Programs were updated by the Canadian Food Inspection Agency in 2017. The Yukon voluntary certification program is currently in the process of incorporating the new and updated standards by the required date of December 31, 2017. An updated Yukon program document will be posted on this site by end of this year.)
Testing from 2001-2017 shows that the Yukon has disease free herds.
Chronic Wasting Disease Links
snip...see;
MONDAY, MAY 29, 2017
Canada CCA optimistic over potential for revisions to OIE criteria for BSE negligible risk
LOL!
Friday, February 20, 2015
A BSE CANADIAN COW MAD COW UPDATE Transcript - Briefing (February 18, 2015)
SATURDAY, FEBRUARY 14, 2015
Canadian Food Inspection Agency Confirms Bovine Spongiform Encephalopathy (BSE) in Alberta
FRIDAY, JANUARY 10, 2014
USDA AUDIT ON CANADA'S MEAT INSPECTION DISTURBING (pot calling kettle black again)
Tuesday, May 21, 2013
Canada, USA, Bad feed, mad cows: Why we know three BSE cases had a common origin and why the SSS policy is in full force $$$
Thursday, January 17, 2013
Canada, U.S. agree on animal-disease measures to protect trade, while reducing human and animal health protection.
Sunday, December 2, 2012
CANADA 19 cases of mad cow disease SCENARIO 4: ‘WE HAD OUR CHANCE AND WE BLEW IT’
Tuesday, October 2, 2012
Canadian veterinarian fined after approving banned BSE high risk cattle for export to U.S.A.
Saturday, January 21, 2012
Quick facts about mad cow disease
Friday, March 4, 2011.
Alberta dairy cow found with mad cow disease.
Thursday, February 10, 2011.
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHY REPORT UPDATE CANADA FEBRUARY 2011 and how to hide mad cow disease in Canada Current as of: 2011-01-31.
Wednesday, December 22, 2010.
Manitoba veterinarian has been fined $10,000 for falsifying certification documents for U.S. bound cattle and what about mad cow disease?
Thursday, August 19, 2010.
REPORT ON THE INVESTIGATION OF THE SEVENTEENTH CASE OF BOVINE SPONGIFORM ENCEPHALOPATHY (BSE) IN CANADA.
Wednesday, August 11, 2010.
REPORT ON THE INVESTIGATION OF THE SIXTEENTH CASE OF BOVINE SPONGIFORM ENCEPHALOPATHY (BSE) IN CANADA.
Increased Atypical Scrapie Detections.
Press reports indicate that increased surveillance is catching what otherwise would have been unreported findings of atypical scrapie in sheep. In 2009, five new cases have been reported in Quebec, Ontario, Alberta, and Saskatchewan. With the exception of Quebec, all cases have been diagnosed as being the atypical form found in older animals. Canada encourages producers to join its voluntary surveillance program in order to gain scrapie-free status. The World Animal Health will not classify Canada as scrapie-free until no new cases are reported for seven years. The Canadian Sheep Federation is calling on the government to fund a wider surveillance program in order to establish the level of prevalence prior to setting an eradication date. Besides long-term testing, industry is calling for a compensation program for farmers who report unusual deaths in their flocks.
SUNDAY, JULY 27, 2008
Docket No. 03-080-1 -- USDA ISSUES PROPOSED RULE TO ALLOW LIVE ANIMAL IMPORTS FROM CANADA
CANADA MBM LIVE CATTLE BSE TSE PRION TO USA
Date: Sat, 14 Jun 2003 02:23:12 +0200
OIG REPORT ON IMPORTS FROM CANADA
*** Subject: USA CJD, BSE, SCRAPIE, CWD, TSE PRION END OF YEAR REPORTS 2017
TUESDAY, DECEMBER 12, 2017
Chronic Wasting Disease CWD TSE Prion (aka mad deer disease) Update USA December 14, 2017
TUESDAY, DECEMBER 12, 2017
Bovine Spongiform Encephalopathy BSE TSE Prion (aka mad cow disease) Report December 14, 2017 2017
TUESDAY, DECEMBER 12, 2017
SCRAPIE TSE PRION UPDATE USA DECEMBER 14, 2017
TUESDAY, DECEMBER 12, 2017
Creutzfeldt Jakob Disease CJD National Prion Disease Pathology Surveillance Center Cases Examined to December 14, 2017
Tuesday, December 12, 2017
Neuropathology of iatrogenic Creutzfeldt–Jakob disease and immunoassay of French cadaver-sourced growth hormone batches suggest possible transmission of tauopathy and long incubation periods for the transmission of Abeta pathology
the tse prion aka mad cow type disease is not your normal pathogen.
The TSE prion disease survives ashing to 600 degrees celsius, that’s around 1112 degrees farenheit.
you cannot cook the TSE prion disease out of meat.
you can take the ash and mix it with saline and inject that ash into a mouse, and the mouse will go down with TSE.
Prion Infected Meat-and-Bone Meal Is Still Infectious after Biodiesel Production as well.
the TSE prion agent also survives Simulated Wastewater Treatment Processes.
IN fact, you should also know that the TSE Prion agent will survive in the environment for years, if not decades.
you can bury it and it will not go away.
The TSE agent is capable of infected your water table i.e. Detection of protease-resistant cervid prion protein in water from a CWD-endemic area.
it’s not your ordinary pathogen you can just cook it out and be done with.
that’s what’s so worrisome about Iatrogenic mode of transmission, a simple autoclave will not kill this TSE prion agent.
1: J Neurol Neurosurg Psychiatry 1994 Jun;57(6):757-8
Transmission of Creutzfeldt-Jakob disease to a chimpanzee by electrodes contaminated during neurosurgery.
Gibbs CJ Jr, Asher DM, Kobrine A, Amyx HL, Sulima MP, Gajdusek DC.
Laboratory of Central Nervous System Studies, National Institute of
Neurological Disorders and Stroke, National Institutes of Health,
Bethesda, MD 20892.
Stereotactic multicontact electrodes used to probe the cerebral cortex of a middle aged woman with progressive dementia were previously implicated in the accidental transmission of Creutzfeldt-Jakob disease (CJD) to two younger patients. The diagnoses of CJD have been confirmed for all three cases. More than two years after their last use in humans, after three cleanings and repeated sterilisation in ethanol and formaldehyde vapour, the electrodes were implanted in the cortex of a chimpanzee. Eighteen months later the animal became ill with CJD. This finding serves to re-emphasise the potential danger posed by reuse of instruments contaminated with the agents of spongiform encephalopathies, even after scrupulous attempts to clean them.
PMID: 8006664 [PubMed - indexed for MEDLINE]
New studies on the heat resistance of hamster-adapted scrapie agent: Threshold survival after ashing at 600°C suggests an inorganic template of replication
Prion Infected Meat-and-Bone Meal Is Still Infectious after Biodiesel Production
Detection of protease-resistant cervid prion protein in water from a CWD-endemic area
A Quantitative Assessment of the Amount of Prion Diverted to Category 1 Materials and Wastewater During Processing
Rapid assessment of bovine spongiform encephalopathy prion inactivation by heat treatment in yellow grease produced in the industrial manufacturing process of meat and bone meals
PPo4-4:
Survival and Limited Spread of TSE Infectivity after Burial
URINE
SUNDAY, JULY 16, 2017
*** Temporal patterns of chronic wasting disease prion excretion in three cervid species ***
FRIDAY, NOVEMBER 24, 2017
Norwegian Food Safety Authority makes changes to measures to limit the spread of disease Skrantesjuke (CWD) in deer wildlife
TITLE: PATHOLOGICAL FEATURES OF CHRONIC WASTING DISEASE IN REINDEER AND DEMONSTRATION OF HORIZONTAL TRANSMISSION
*** DECEMBER 2016 CDC EMERGING INFECTIOUS DISEASE JOURNAL CWD HORIZONTAL TRANSMISSION
Using in vitro prion replication for high sensitive detection of prions and prionlike proteins and for understanding mechanisms of transmission.
Claudio Soto Mitchell Center for Alzheimer's diseases and related Brain disorders, Department of Neurology, University of Texas Medical School at Houston.
***Recently, we have been using PMCA to study the role of environmental prion contamination on the horizontal spreading of TSEs. These experiments have focused on the study of the interaction of prions with plants and environmentally relevant surfaces. Our results show that plants (both leaves and roots) bind tightly to prions present in brain extracts and excreta (urine and feces) and retain even small quantities of PrPSc for long periods of time. Strikingly, ingestion of prioncontaminated leaves and roots produced disease with a 100% attack rate and an incubation period not substantially longer than feeding animals directly with scrapie brain homogenate. Furthermore, plants can uptake prions from contaminated soil and transport them to different parts of the plant tissue (stem and leaves). Similarly, prions bind tightly to a variety of environmentally relevant surfaces, including stones, wood, metals, plastic, glass, cement, etc. Prion contaminated surfaces efficiently transmit prion disease when these materials were directly injected into the brain of animals and strikingly when the contaminated surfaces were just placed in the animal cage. These findings demonstrate that environmental materials can efficiently bind infectious prions and act as carriers of infectivity, suggesting that they may play an important role in the horizontal transmission of the disease.
Since its invention 13 years ago, PMCA has helped to answer fundamental questions of prion propagation and has broad applications in research areas including the food industry, blood bank safety and human and veterinary disease diagnosis.
In conclusion, the results in the current study indicate that removal of furniture that had been in contact with scrapie-infected animals should be recommended, particularly since cleaning and decontamination may not effectively remove scrapie infectivity (31), even though infectivity declines considerably if the pasture and the field furniture have not been in contact with scrapie-infected sheep for several months. As sPMCA failed to detect PrPSc in furniture that was subjected to weathering, even though exposure led to infection in sheep, this method may not always be reliable in predicting the risk of scrapie infection through environmental contamination. These results suggest that the VRQ/VRQ sheep model may be more sensitive than sPMCA for the detection of environmentally associated scrapie, and suggest that extremely low levels of scrapie contamination are able to cause infection in susceptible sheep genotypes.
Keywords: classical scrapie, prion, transmissible spongiform encephalopathy, sheep, field furniture, reservoir, serial protein misfolding cyclic amplification
Wednesday, December 16, 2015
*** Objects in contact with classical scrapie sheep act as a reservoir for scrapie transmission ***
*** Infectious agent of sheep scrapie may persist in the environment for at least 16 years ***
Gudmundur Georgsson1, Sigurdur Sigurdarson2 and Paul Brown3
with CWD TSE Prions, I am not sure there is any absolute yet, other than what we know with transmission studies, and we know tse prion kill, and tse prion are bad. science shows to date, that indeed soil, dirt, some better than others, can act as a carrier. same with objects, farm furniture. take it with how ever many grains of salt you wish, or not. if load factor plays a role in the end formula, then everything should be on the table, in my opinion...tss
Oral Transmissibility of Prion Disease Is Enhanced by Binding to Soil Particles
Transmissible spongiform encephalopathies (TSEs) are a group of incurable neurological diseases likely caused by a misfolded form of the prion protein. TSEs include scrapie in sheep, bovine spongiform encephalopathy (‘‘mad cow’’ disease) in cattle, chronic wasting disease in deer and elk, and Creutzfeldt-Jakob disease in humans. Scrapie and chronic wasting disease are unique among TSEs because they can be transmitted between animals, and the disease agents appear to persist in environments previously inhabited by infected animals. Soil has been hypothesized to act as a reservoir of infectivity and to bind the infectious agent. In the current study, we orally dosed experimental animals with a common clay mineral, montmorillonite, or whole soils laden with infectious prions, and compared the transmissibility to unbound agent. We found that prions bound to montmorillonite and whole soils remained orally infectious, and, in most cases, increased the oral transmission of disease compared to the unbound agent. The results presented in this study suggest that soil may contribute to environmental spread of TSEs by increasing the transmissibility of small amounts of infectious agent in the environment.
Wednesday, December 16, 2015
Objects in contact with classical scrapie sheep act as a reservoir for scrapie transmission
The sources of dust borne prions are unknown but it seems reasonable to assume that faecal, urine, skin, parturient material and saliva-derived prions may contribute to this mobile environmental reservoir of infectivity. This work highlights a possible transmission route for scrapie within the farm environment, and this is likely to be paralleled in CWD which shows strong similarities with scrapie in terms of prion dissemination and disease transmission. The data indicate that the presence of scrapie prions in dust is likely to make the control of these diseases a considerable challenge.
>>>Particle-associated PrPTSE molecules may migrate from locations of deposition via transport processes affecting soil particles, including entrainment in and movement with air and overland flow. <<<
Fate of Prions in Soil: A Review
Christen B. Smith, Clarissa J. Booth, and Joel A. Pedersen*
Several reports have shown that prions can persist in soil for several years. Significant interest remains in developing methods that could be applied to degrade PrPTSE in naturally contaminated soils. Preliminary research suggests that serine proteases and the microbial consortia in stimulated soils and compost may partially degrade PrPTSE. Transition metal oxides in soil (viz. manganese oxide) may also mediate prion inactivation. Overall, the effect of prion attachment to soil particles on its persistence in the environment is not well understood, and additional study is needed to determine its implications on the environmental transmission of scrapie and CWD.
P.161: Prion soil binding may explain efficient horizontal CWD transmission
Conclusion. Silty clay loam exhibits highly efficient prion binding, inferring a durable environmental reservoir, and an efficient mechanism for indirect horizontal CWD transmission.
>>>Another alternative would be an absolute prohibition on the movement of deer within the state for any purpose. While this alternative would significantly reduce the potential spread of CWD, it would also have the simultaneous effect of preventing landowners and land managers from implementing popular management strategies involving the movement of deer, and would deprive deer breeders of the ability to engage in the business of buying and selling breeder deer. Therefore, this alternative was rejected because the department determined that it placed an avoidable burden on the regulated community.<<<
Wednesday, December 16, 2015
Objects in contact with classical scrapie sheep act as a reservoir for scrapie transmission
Timm Konold1*, Stephen A. C. Hawkins2, Lisa C. Thurston3, Ben C. Maddison4, Kevin C. Gough5, Anthony Duarte1 and Hugh A. Simmons1
1 Animal Sciences Unit, Animal and Plant Health Agency Weybridge, Addlestone, UK, 2 Pathology Department, Animal and Plant Health Agency Weybridge, Addlestone, UK, 3 Surveillance and Laboratory Services, Animal and Plant Health Agency Penrith, Penrith, UK, 4 ADAS UK, School of Veterinary Medicine and Science, University of Nottingham, Sutton Bonington, UK, 5 School of Veterinary Medicine and Science, University of Nottingham, Sutton Bonington, UK
Classical scrapie is an environmentally transmissible prion disease of sheep and goats. Prions can persist and remain potentially infectious in the environment for many years and thus pose a risk of infecting animals after re-stocking. In vitro studies using serial protein misfolding cyclic amplification (sPMCA) have suggested that objects on a scrapie affected sheep farm could contribute to disease transmission. This in vivo study aimed to determine the role of field furniture (water troughs, feeding troughs, fencing, and other objects that sheep may rub against) used by a scrapie-infected sheep flock as a vector for disease transmission to scrapie-free lambs with the prion protein genotype VRQ/VRQ, which is associated with high susceptibility to classical scrapie. When the field furniture was placed in clean accommodation, sheep became infected when exposed to either a water trough (four out of five) or to objects used for rubbing (four out of seven). This field furniture had been used by the scrapie-infected flock 8 weeks earlier and had previously been shown to harbor scrapie prions by sPMCA. Sheep also became infected (20 out of 23) through exposure to contaminated field furniture placed within pasture not used by scrapie-infected sheep for 40 months, even though swabs from this furniture tested negative by PMCA. This infection rate decreased (1 out of 12) on the same paddock after replacement with clean field furniture. Twelve grazing sheep exposed to field furniture not in contact with scrapie-infected sheep for 18 months remained scrapie free. The findings of this study highlight the role of field furniture used by scrapie-infected sheep to act as a reservoir for disease re-introduction although infectivity declines considerably if the field furniture has not been in contact with scrapie-infected sheep for several months. PMCA may not be as sensitive as VRQ/VRQ sheep to test for environmental contamination.
Classical scrapie is an environmentally transmissible disease because it has been reported in naïve, supposedly previously unexposed sheep placed in pastures formerly occupied by scrapie-infected sheep (4, 19, 20). Although the vector for disease transmission is not known, soil is likely to be an important reservoir for prions (2) where – based on studies in rodents – prions can adhere to minerals as a biologically active form (21) and remain infectious for more than 2 years (22). Similarly, chronic wasting disease (CWD) has re-occurred in mule deer housed in paddocks used by infected deer 2 years earlier, which was assumed to be through foraging and soil consumption (23).
Our study suggested that the risk of acquiring scrapie infection was greater through exposure to contaminated wooden, plastic, and metal surfaces via water or food troughs, fencing, and hurdles than through grazing. Drinking from a water trough used by the scrapie flock was sufficient to cause infection in sheep in a clean building. Exposure to fences and other objects used for rubbing also led to infection, which supported the hypothesis that skin may be a vector for disease transmission (9). The risk of these objects to cause infection was further demonstrated when 87% of 23 sheep presented with PrPSc in lymphoid tissue after grazing on one of the paddocks, which contained metal hurdles, a metal lamb creep and a water trough in contact with the scrapie flock up to 8 weeks earlier, whereas no infection had been demonstrated previously in sheep grazing on this paddock, when equipped with new fencing and field furniture. When the contaminated furniture and fencing were removed, the infection rate dropped significantly to 8% of 12 sheep, with soil of the paddock as the most likely source of infection caused by shedding of prions from the scrapie-infected sheep in this paddock up to a week earlier.
This study also indicated that the level of contamination of field furniture sufficient to cause infection was dependent on two factors: stage of incubation period and time of last use by scrapie-infected sheep. Drinking from a water trough that had been used by scrapie sheep in the predominantly pre-clinical phase did not appear to cause infection, whereas infection was shown in sheep drinking from the water trough used by scrapie sheep in the later stage of the disease. It is possible that contamination occurred through shedding of prions in saliva, which may have contaminated the surface of the water trough and subsequently the water when it was refilled. Contamination appeared to be sufficient to cause infection only if the trough was in contact with sheep that included clinical cases. Indeed, there is an increased risk of bodily fluid infectivity with disease progression in scrapie (24) and CWD (25) based on PrPSc detection by sPMCA. Although ultraviolet light and heat under natural conditions do not inactivate prions (26), furniture in contact with the scrapie flock, which was assumed to be sufficiently contaminated to cause infection, did not act as vector for disease if not used for 18 months, which suggest that the weathering process alone was sufficient to inactivate prions.
PrPSc detection by sPMCA is increasingly used as a surrogate for infectivity measurements by bioassay in sheep or mice. In this reported study, however, the levels of PrPSc present in the environment were below the limit of detection of the sPMCA method, yet were still sufficient to cause infection of in-contact animals. In the present study, the outdoor objects were removed from the infected flock 8 weeks prior to sampling and were positive by sPMCA at very low levels (2 out of 37 reactions). As this sPMCA assay also yielded 2 positive reactions out of 139 in samples from the scrapie-free farm, the sPMCA assay could not detect PrPSc on any of the objects above the background of the assay. False positive reactions with sPMCA at a low frequency associated with de novo formation of infectious prions have been reported (27, 28). This is in contrast to our previous study where we demonstrated that outdoor objects that had been in contact with the scrapie-infected flock up to 20 days prior to sampling harbored PrPSc that was detectable by sPMCA analysis [4 out of 15 reactions (12)] and was significantly more positive by the assay compared to analogous samples from the scrapie-free farm. This discrepancy could be due to the use of a different sPMCA substrate between the studies that may alter the efficiency of amplification of the environmental PrPSc. In addition, the present study had a longer timeframe between the objects being in contact with the infected flock and sampling, which may affect the levels of extractable PrPSc. Alternatively, there may be potentially patchy contamination of this furniture with PrPSc, which may have been missed by swabbing. The failure of sPMCA to detect CWD-associated PrP in saliva from clinically affected deer despite confirmation of infectivity in saliva-inoculated transgenic mice was associated with as yet unidentified inhibitors in saliva (29), and it is possible that the sensitivity of sPMCA is affected by other substances in the tested material. In addition, sampling of amplifiable PrPSc and subsequent detection by sPMCA may be more difficult from furniture exposed to weather, which is supported by the observation that PrPSc was detected by sPMCA more frequently in indoor than outdoor furniture (12). A recent experimental study has demonstrated that repeated cycles of drying and wetting of prion-contaminated soil, equivalent to what is expected under natural weathering conditions, could reduce PMCA amplification efficiency and extend the incubation period in hamsters inoculated with soil samples (30). This seems to apply also to this study even though the reduction in infectivity was more dramatic in the sPMCA assays than in the sheep model. Sheep were not kept until clinical end-point, which would have enabled us to compare incubation periods, but the lack of infection in sheep exposed to furniture that had not been in contact with scrapie sheep for a longer time period supports the hypothesis that prion degradation and subsequent loss of infectivity occurs even under natural conditions.
In conclusion, the results in the current study indicate that removal of furniture that had been in contact with scrapie-infected animals should be recommended, particularly since cleaning and decontamination may not effectively remove scrapie infectivity (31), even though infectivity declines considerably if the pasture and the field furniture have not been in contact with scrapie-infected sheep for several months. As sPMCA failed to detect PrPSc in furniture that was subjected to weathering, even though exposure led to infection in sheep, this method may not always be reliable in predicting the risk of scrapie infection through environmental contamination. These results suggest that the VRQ/VRQ sheep model may be more sensitive than sPMCA for the detection of environmentally associated scrapie, and suggest that extremely low levels of scrapie contamination are able to cause infection in susceptible sheep genotypes.
Keywords: classical scrapie, prion, transmissible spongiform encephalopathy, sheep, field furniture, reservoir, serial protein misfolding cyclic amplification
Wednesday, December 16, 2015
*** Objects in contact with classical scrapie sheep act as a reservoir for scrapie transmission ***
*** Infectious agent of sheep scrapie may persist in the environment for at least 16 years ***
Gudmundur Georgsson1, Sigurdur Sigurdarson2 and Paul Brown3
Rethinking Major grain organizations opposition to CFIA's control zone approach to Chronic Wasting CWD TSE Prion Mad Deer Type Disease 2017?
*** Chronic Wasting Disease CWD TSE Prion aka Mad Deer Disease and the Real Estate Market Land Values ***
*** After a natural route of exposure, 100% of WTD were susceptible to scrapie.
PO-039: A comparison of scrapie and chronic wasting disease in white-tailed deer
Justin Greenlee, Jodi Smith, Eric Nicholson US Dept. Agriculture; Agricultural Research Service, National Animal Disease Center; Ames, IA USA
White-tailed deer are susceptible to the agent of sheep scrapie by intracerebral inoculation
snip...
It is unlikely that CWD will be eradicated from free-ranging cervids, and the disease is likely to continue to spread geographically [10]. However, the potential that white-tailed deer may be susceptible to sheep scrapie by a natural route presents an additional confounding factor to halting the spread of CWD. This leads to the additional speculations that
1) infected deer could serve as a reservoir to infect sheep with scrapie offering challenges to scrapie eradication efforts and
2) CWD spread need not remain geographically confined to current endemic areas, but could occur anywhere that sheep with scrapie and susceptible cervids cohabitate.
This work demonstrates for the first time that white-tailed deer are susceptible to sheep scrapie by intracerebral inoculation with a high attack rate and that the disease that results has similarities to CWD. These experiments will be repeated with a more natural route of inoculation to determine the likelihood of the potential transmission of sheep scrapie to white-tailed deer. If scrapie were to occur in white-tailed deer, results of this study indicate that it would be detected as a TSE, but may be difficult to differentiate from CWD without in-depth biochemical analysis.
2012
PO-039: A comparison of scrapie and chronic wasting disease in white-tailed deer
Justin Greenlee, Jodi Smith, Eric Nicholson US Dept. Agriculture; Agricultural Research Service, National Animal Disease Center; Ames, IA USA
snip...
The results of this study suggest that there are many similarities in the manifestation of CWD and scrapie in WTD after IC inoculation including early and widespread presence of PrPSc in lymphoid tissues, clinical signs of depression and weight loss progressing to wasting, and an incubation time of 21-23 months. Moreover, western blots (WB) done on brain material from the obex region have a molecular profile similar to CWD and distinct from tissues of the cerebrum or the scrapie inoculum. However, results of microscopic and IHC examination indicate that there are differences between the lesions expected in CWD and those that occur in deer with scrapie: amyloid plaques were not noted in any sections of brain examined from these deer and the pattern of immunoreactivity by IHC was diffuse rather than plaque-like.
*** After a natural route of exposure, 100% of WTD were susceptible to scrapie.
Deer developed clinical signs of wasting and mental depression and were necropsied from 28 to 33 months PI. Tissues from these deer were positive for PrPSc by IHC and WB. Similar to IC inoculated deer, samples from these deer exhibited two different molecular profiles: samples from obex resembled CWD whereas those from cerebrum were similar to the original scrapie inoculum. On further examination by WB using a panel of antibodies, the tissues from deer with scrapie exhibit properties differing from tissues either from sheep with scrapie or WTD with CWD. Samples from WTD with CWD or sheep with scrapie are strongly immunoreactive when probed with mAb P4, however, samples from WTD with scrapie are only weakly immunoreactive. In contrast, when probed with mAb’s 6H4 or SAF 84, samples from sheep with scrapie and WTD with CWD are weakly immunoreactive and samples from WTD with scrapie are strongly positive. This work demonstrates that WTD are highly susceptible to sheep scrapie, but on first passage, scrapie in WTD is differentiable from CWD.
2011
*** After a natural route of exposure, 100% of white-tailed deer were susceptible to scrapie.
TUESDAY, MARCH 28, 2017
*** Passage of scrapie to deer results in a new phenotype upon return passage to sheep ***
CWD TO PIGS
Research Project: TRANSMISSION, DIFFERENTIATION, AND PATHOBIOLOGY OF TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES
Location: Virus and Prion Research
Title: Disease-associated prion protein detected in lymphoid tissues from pigs challenged with the agent of chronic wasting disease
Author item Moore, Sarah item Kunkle, Robert item Kondru, Naveen item Manne, Sireesha item Smith, Jodi item Kanthasamy, Anumantha item West Greenlee, M item Greenlee, Justin
Submitted to: Prion Publication Type: Abstract Only Publication Acceptance Date: 3/15/2017 Publication Date: N/A Citation: N/A Interpretive Summary:
Technical Abstract: Aims: Chronic wasting disease (CWD) is a naturally-occurring, fatal neurodegenerative disease of cervids. We previously demonstrated that disease-associated prion protein (PrPSc) can be detected in the brain and retina from pigs challenged intracranially or orally with the CWD agent. In that study, neurological signs consistent with prion disease were observed only in one pig: an intracranially challenged pig that was euthanized at 64 months post-challenge. The purpose of this study was to use an antigen-capture immunoassay (EIA) and real-time quaking-induced conversion (QuIC) to determine whether PrPSc is present in lymphoid tissues from pigs challenged with the CWD agent.
Methods: At two months of age, crossbred pigs were challenged by the intracranial route (n=20), oral route (n=19), or were left unchallenged (n=9). At approximately 6 months of age, the time at which commercial pigs reach market weight, half of the pigs in each group were culled (<6 challenge="" groups="" month="" pigs="" remaining="" the="">6 month challenge groups) were allowed to incubate for up to 73 months post challenge (mpc). The retropharyngeal lymph node (RPLN) was screened for the presence of PrPSc by EIA and immunohistochemistry (IHC). The RPLN, palatine tonsil, and mesenteric lymph node (MLN) from 6-7 pigs per challenge group were also tested using EIA and QuIC.
Results: PrPSc was not detected by EIA and IHC in any RPLNs. All tonsils and MLNs were negative by IHC, though the MLN from one pig in the oral <6 5="" 6="" at="" by="" detected="" eia.="" examined="" group="" in="" intracranial="" least="" lymphoid="" month="" months="" of="" one="" pigs="" positive="" prpsc="" quic="" the="" tissues="" was="">6 months group, 5/6 pigs in the oral <6 4="" and="" group="" months="" oral="">6 months group. Overall, the MLN was positive in 14/19 (74%) of samples examined, the RPLN in 8/18 (44%), and the tonsil in 10/25 (40%). Conclusions:
This study demonstrates that PrPSc accumulates in lymphoid tissues from pigs challenged intracranially or orally with the CWD agent, and can be detected as early as 4 months after challenge.
CWD-infected pigs rarely develop clinical disease and if they do, they do so after a long incubation period. This raises the possibility that CWD-infected pigs could shed prions into their environment long before they develop clinical disease.
Furthermore, lymphoid tissues from CWD-infected pigs could present a potential source of CWD infectivity in the animal and human food chains.
CONFIDENTIAL
EXPERIMENTAL PORCINE SPONGIFORM ENCEPHALOPATHY
While this clearly is a cause for concern we should not jump to the conclusion that this means that pigs will necessarily be infected by bone and meat meal fed by the oral route as is the case with cattle. ...
we cannot rule out the possibility that unrecognised subclinical spongiform encephalopathy could be present in British pigs though there is no evidence for this: only with parenteral/implantable pharmaceuticals/devices is the theoretical risk to humans of sufficient concern to consider any action.
Our records show that while some use is made of porcine materials in medicinal products, the only products which would appear to be in a hypothetically ''higher risk'' area are the adrenocorticotrophic hormone for which the source material comes from outside the United Kingdom, namely America China Sweden France and Germany. The products are manufactured by Ferring and Armour. A further product, ''Zenoderm Corium implant'' manufactured by Ethicon, makes use of porcine skin - which is not considered to be a ''high risk'' tissue, but one of its uses is described in the data sheet as ''in dural replacement''. This product is sourced from the United Kingdom.....
snip...see much more here ;
WEDNESDAY, APRIL 05, 2017
Disease-associated prion protein detected in lymphoid tissues from pigs challenged with the agent of chronic wasting disease
WEDNESDAY, APRIL 05, 2017
*** Disease-associated prion protein detected in lymphoid tissues from pigs challenged with the agent of chronic wasting disease ***
cattle are highly susceptible to white-tailed deer CWD and mule deer CWD
***In contrast, cattle are highly susceptible to white-tailed deer CWD and mule deer CWD in experimental conditions but no natural CWD infections in cattle have been reported (Sigurdson, 2008; Hamir et al., 2006). It is not known how susceptible humans are to CWD but given that the prion can be present in muscle, it is likely that humans have been exposed to the agent via consumption of venison (Sigurdson, 2008). Initial experimental research, however, suggests that human susceptibility to CWD is low and there may be a robust species barrier for CWD transmission to humans (Sigurdson, 2008). It is apparent, though, that CWD is affecting wild and farmed cervid populations in endemic areas with some deer populations decreasing as a result.
SNIP...
price of prion poker goes up for cwd to cattle;
Monday, April 04, 2016
*** Limited amplification of chronic wasting disease prions in the peripheral tissues of intracerebrally inoculated cattle ***
*** The potential impact of prion diseases on human health was greatly magnified by the recognition that interspecies transfer of BSE to humans by beef ingestion resulted in vCJD. While changes in animal feed constituents and slaughter practices appear to have curtailed vCJD, there is concern that CWD of free-ranging deer and elk in the U.S. might also cross the species barrier. Thus, consuming venison could be a source of human prion disease. Whether BSE and CWD represent interspecies scrapie transfer or are newly arisen prion diseases is unknown. Therefore, the possibility of transmission of prion disease through other food animals cannot be ruled out. There is evidence that vCJD can be transmitted through blood transfusion. There is likely a pool of unknown size of asymptomatic individuals infected with vCJD, and there may be asymptomatic individuals infected with the CWD equivalent. These circumstances represent a potential threat to blood, blood products, and plasma supplies.
SATURDAY, JULY 29, 2017
Risk Advisory Opinion: Potential Human Health Risks from Chronic Wasting Disease CFIA, PHAC, HC (HPFB and FNIHB), INAC, Parks Canada, ECCC and AAFC
SAWCorp CWD Test
PLEASE BE AWARE, SOME ARE PUSHING TO USE SAWCorp CWD Test TO ASSURE YOUR CERVID IS CWD FREE, SAWCorp CWD Test HAS _NOT_ BEEN APPROVED BY APHIS !!! IMPORTANT: SAWCorp CWD Test is Not APHIS Approved
USDA Animal and Plant Health Inspection Service sent this bulletin at 11/18/2016 11:43 AM EST
SAWCorp, a private company, recently issued a press release launching a new, patented live-animal blood test for the detection of chronic wasting disease (CWD) in cervids. A subsequent press release from the same company stated that the USDA is reviewing the test for use in the CWD program. USDA’s Animal and Plant Health Inspection Service (APHIS) does not recognize protein misfolding cyclic amplification (PMCA) prion blood tests as an official test for CWD, bovine spongiform encephalopathy,or scrapie. By definition, an official CWD test is, “Any test for the diagnosis of CWD approved by the Administrator and conducted in a laboratory approved by the Administrator in accordance with §55.8 of this part” (9 CFR Part 55).
The criteria necessary for approval as an official CWD test includes a standardized test protocol, data to support reproducibility, data to support suitability, and data to support the sensitivity and specificity of the test. While APHIS supports emerging technologies, no company has submitted the data needed for APHIS to evaluate the PMCA prion blood test. In addition, APHIS is aware of no peer-reviewed scientific publications that establish the efficacy of PMCA as a detection method for CWD in cervid blood. If producers elect to use a PMCA test, APHIS will consider positive results to be “suspect” cases that must be confirmed using an official CWD test. APHIS will not recognize negative or “not detected” PMCA test results for herd certification or interstate movement purposes.
Subject: cwd genetic susceptibility
Genetic susceptibility to chronic wasting disease in free-ranging white-tailed deer: Complement component C1q and Prnp polymorphisms§
Julie A. Blanchong a, *, Dennis M. Heisey b , Kim T. Scribner c , Scot V. Libants d , Chad Johnson e , Judd M. Aiken e , Julia A. Langenberg f , Michael D. Samuel g
snip...
Identifying the genetic basis for heterogeneity in disease susceptibility or progression can improve our understanding of individual variation in disease susceptibility in both free-ranging and captive populations. What this individual variation in disease susceptibility means for the trajectory of disease in a population, however, is not straightforward. For example, the greater, but not complete, resistance to CWD in deer with at least one Serine (S) at amino acid 96 of the Prnp gene appears to be associated with slower progression of disease (e.g., Johnson et al., 2006; Keane et al., 2008a). If slower disease progression results in longer-lived, infected deer with longer periods of infectiousness, resistance may lead to increased disease transmission rates, higher prion concentrations in the environment, and increased prevalence, as has been observed in some captive deer herds (Miller et al., 2006; Keane et al., 2008a). Alternatively, if the slower progression of disease in resistant deer is not associated with longer periods of infectiousness, but might instead indicate a higher dose of PrPCWD is required for infection, transmission rates in the population could decline especially if, as in Wisconsin, deer suffer high rates of mortality from other sources (e.g., hunting). Clearly, determining the relationship between genetic susceptibility to infection, dose requirements, disease progression, and the period of PrPCWD infectiousness are key components for understanding the consequences of CWD to free-ranging populations.
Prion protein gene sequence and chronic wasting disease susceptibility in white-tailed deer (Odocoileus virginianus)
Adam L Brandt,1 Amy C Kelly,1 Michelle L Green,1,2 Paul Shelton,3 Jan Novakofski,2,* and Nohra E Mateus-Pinilla1,2 Author information ► Article notes ► Copyright and License information ►
The sequence of the prion protein gene (PRNP) affects susceptibility to spongiform encephalopathies, or prion diseases in many species. In white-tailed deer, both coding and non-coding single nucleotide polymorphisms have been identified in this gene that correlate to chronic wasting disease (CWD) susceptibility. Previous studies examined individual nucleotide or amino acid mutations; here we examine all nucleotide polymorphisms and their combined effects on CWD. A 626 bp region of PRNP was examined from 703 free-ranging white-tailed deer. Deer were sampled between 2002 and 2010 by hunter harvest or government culling in Illinois and Wisconsin. Fourteen variable nucleotide positions were identified (4 new and 10 previously reported). We identified 68 diplotypes comprised of 24 predicted haplotypes, with the most common diplotype occurring in 123 individuals. Diplotypes that were found exclusively among positive or negative animals were rare, each occurring in less than 1% of the deer studied. Only one haplotype (C, odds ratio 0.240) and 2 diplotypes (AC and BC, odds ratios of 0.161 and 0.108 respectively) has significant associations with CWD resistance. Each contains mutations (one synonymous nucleotide 555C/T and one nonsynonymous nucleotide 286G/A) at positions reported to be significantly associated with reduced CWD susceptibility. Results suggest that deer populations with higher frequencies of haplotype C or diplotypes AC and BC might have a reduced risk for CWD infection – while populations with lower frequencies may have higher risk for infection. Understanding the genetic basis of CWD has improved our ability to assess herd susceptibility and direct management efforts within CWD infected areas.
KEYWORDS: CWD, diplotype, G96S, PRNP, prion, synonymous polymorphism, haplotype
snip...
A solid understanding of the genetics of CWD in white-tailed deer is vital to improve management of CWD on the landscape. Most TSEs are found in domestic or captive animals where management of infected individuals is feasible. For example, scrapie infected flocks can be handled through a process generally involving genetic testing, removal and destruction of infected or suspect animals, followed by decontamination of facilities and equipment.55Containment of free ranging deer in wild populations potentially infected with CWD and decontamination of the environment is not reasonably possible. The long term effects of CWD are not yet known but it is conceivable that an unmanaged infected population would be gradually extirpated as the disease progresses56,57 or at least reduced to low densities with high disease prevalence.58,59 Either outcome would have severe ecological effects (e.g., deer play a major role in affecting plant communities60 and as a prey source61,62) as well as negative economic impacts to hunting. Overall disease prevalence has remained at relatively low levels in Illinois compared to Wisconsin.11 It is important to note that at the time of sampling, CWD had been found in 6 Illinois counties and has since been detected in 14.9Complete eradication of CWD among free ranging white-tailed deer may not be possible; however, an active containment effort in Illinois appears to have prevented significant increases in prevalence.9,11,12 Further examination of PRNP haplotype and diplotype frequencies across northern Illinois and southern Wisconsin in conjunction with population structure and movement45,63,64 will be useful in identifying localities with greater or reduced susceptibility risk. Effectiveness of CWD containment efforts can be aided through genetic testing and redirecting management resources.
***at present, no cervid PrP allele conferring absolute resistance to prion infection has been identified.
P-145 Estimating chronic wasting disease resistance in cervids using real time quaking- induced conversion
Nicholas J Haley1, Rachel Rielinqer2, Kristen A Davenport3, W. David Walter4, Katherine I O'Rourke5, Gordon Mitchell6, Juergen A Richt2 1 Department of Microbiology and Immunology, Midwestern University, United States; 2Department of Diagnostic Medicine and Pathobiology, Kansas State University; 3Prion Research Center; Colorado State University; 4U.S. Geological Survey, Pennsylvania Cooperative Fish and Wildlife Research Unit; 5Agricultural Research Service, United States Department of Agriculture; 6Canadian Food Inspection Agency, National and OlE Reference Laboratory for Scrapie and CWD
In mammalian species, the susceptibility to prion diseases is affected, in part, by the sequence of the host's prion protein (PrP). In sheep, a gradation from scrapie susceptible to resistant has been established both in vivo and in vitro based on the amino acids present at PrP positions 136, 154, and 171, which has led to global breeding programs to reduce the prevalence of scrapie in domestic sheep. In cervids, resistance is commonly characterized as a delayed progression of chronic wasting disease (CWD); at present, no cervid PrP allele conferring absolute resistance to prion infection has been identified. To model the susceptibility of various naturally-occurring and hypothetical cervid PrP alleles in vitro, we compared the amplification rates and efficiency of various CWD isolates in recombinant PrPC using real time quaking-induced conversion. We hypothesized that amplification metrics of these isolates in cervid PrP substrates would correlate to in vivo susceptibility - allowing susceptibility prediction for alleles found at 10 frequency in nature, and that there would be an additive effect of multiple resistant codons in hypothetical alleles. Our studies demonstrate that in vitro amplification metrics predict in vivo susceptibility, and that alleles with multiple codons, each influencing resistance independently, do not necessarily contribute additively to resistance. Importantly, we found that the white-tailed deer 226K substrate exhibited the slowest amplification rate among those evaluated, suggesting that further investigation of this allele and its resistance in vivo are warranted to determine if absolute resistance to CWD is possible.
***at present, no cervid PrP allele conferring absolute resistance to prion infection has been identified.
PRION 2016 CONFERENCE TOKYO
''There are no known familial or genetic TSEs of animals, although polymorphisms in the PRNP gene of some species (sheep for example) may influence the length of the incubation period and occurrence of disease.''
c) The commonest form of CJD occurs as a sporadic disease, the cause of which is unknown, although genetic factors (particularly the codon 129 polymorphism in the prion protein gene (PRNP)) influence disease susceptibility. The familial forms of human TSEs (see Box 1) appear to have a solely genetic origin and are closely associated with mutations or insertions in the PRNP gene. Most, but not all, of the familial forms of human TSEs have been transmitted experimentally to animals. There are no known familial or genetic TSEs of animals, although polymorphisms in the PRNP gene of some species (sheep for example) may influence the length of the incubation period and occurrence of disease.
''There are no known familial or genetic TSEs of animals, although polymorphisms in the PRNP gene of some species (sheep for example) may influence the length of the incubation period and occurrence of disease.''
c) The commonest form of CJD occurs as a sporadic disease, the cause of which is unknown, although genetic factors (particularly the codon 129 polymorphism in the prion protein gene (PRNP)) influence disease susceptibility. The familial forms of human TSEs (see Box 1) appear to have a solely genetic origin and are closely associated with mutations or insertions in the PRNP gene. Most, but not all, of the familial forms of human TSEs have been transmitted experimentally to animals. There are no known familial or genetic TSEs of animals, although polymorphisms in the PRNP gene of some species (sheep for example) may influence the length of the incubation period and occurrence of disease.
SUNDAY, DECEMBER 10, 2017
Detection of Prions in Blood of Cervids at the Asymptomatic Stage of Chronic Wasting Disease
The results showed a sensitivity of 100% in animals with very poor body condition that were IHC-positive in both brain and lymph nodes, 96% in asymptomatic deer IHC-positive in brain and lymph nodes and 53% in animals at early stages of infection that were IHC-positive only in lymph nodes. The overall mean diagnostic sensitivity was 79.3% with 100% specificity. These findings show that PMCA might be useful as a blood test for routine, live animal diagnosis of CWD.
MONDAY, SEPTEMBER 25, 2017
Colorado Chronic Wasting Disease CWD TSE Prion Mandatory Submission of test samples in some areas and zoonosis
(ALSO, see the debate and evidence showing the origin of CWD starting in Colorado captive research pen)
Iowa Supreme Court rules law allows quarantine of CWD deer, not land
This is very, very concerning imo.
IF this ruling is upheld as such ;
''The Iowa Supreme Court upheld the district court ruling — saying the law gives the DNR only the authority to quarantine the deer — not the land. The ruling says if the Iowa Legislature wants to expand the quarantine powers as suggested by the DNR, then it is free to do so.''
IF a 'precedent' is set as such, by the Legislature not intervening to expand quarantine powers to the DNR for CWD TSE Prion, and the precedent is set as such that the cervid industry and land there from, once contaminated with the CWD TSE Prion, are free to repopulate, sell the land, etc, imo, this will blow the lid off any containment efforts of this damn disease CWD TSE Prion. The Iowa Supreme Court did not just pass the cwd buck down the road, the Supreme Court of Iowa just threw the whole state of Iowa under the bus at 100 MPH. all those healthy deer, while the litigation was going on, well, they were incubating the cwd tse prion, loading up the land even more, and in the end, 79.8% of those healthy looking deer had CWD TSE Prion. what about the exposure to the other species that come across that land, and then off to some other land? this makes no sense to me, if this is set in stone and the Legislation does not stop it, and stop if fast, any containment of the cwd tse prion will be futile, imo...terry
FRIDAY, JUNE 16, 2017
Iowa Supreme Court rules law allows quarantine of CWD deer, not land
FRIDAY, NOVEMBER 24, 2017
Todd Robbins-Miller President of Minnesota Deer Farmers Association is oblivious to Chronic Wasting CWD TSE PRION DISEASE risk factors
FRIDAY, NOVEMBER 24, 2017
Brain Tanning Hides and CWD Transmissible Spongiform Encephalopathy TSE Prion Disease Risk Factors Warning
EUROPE CWD TSE PRION
What is the risk of chronic wasting disease being introduced into Great Britain? A Qualitative Risk Assessment October 2012
Summary
Chronic wasting disease (CWD) is a highly infectious transmissible spongiform encephalopathy (TSE) that is circulating in the wild and farmed cervid populations in North America. It is the only TSE to be prevalent in free-ranging wild animal populations. A feature of CWD is its ability to spread both directly and indirectly via the contaminated environment where it is able to survive in a bio-available form for many years without any significant decrease in infectivity. Eradication of the disease from wild and farmed or managed cervid populations and the environment is extremely challenging and has not yet been successful.
Currently, there have been no reported cases of CWD or other TSE in deer in Great Britain (GB) or Europe. Given the consequences of CWD observed in North America, it is imperative that GB remains free of the disease. This risk assessment aims to assess the risk of CWD being imported into GB from North America and consequently, consider the risk of exposure and infection within the GB deer population. The assessment focuses on two main routes of entry including importation of animal feed and movement of contaminated clothing, footwear and equipment of tourists, deer hunters and British servicemen between affected areas of North America and GB. It is important to highlight that there are significant data gaps in this assessment. The main conclusions from this assessment are:
Several different animal feed products are imported into GB from North America. These include processed pet foods and consignments of unfinished feed ingredients for use in animal feed. The amount of imported feed, including pet food, that contains cervid protein is unknown and identified as a significant data gap. As non-ruminant animal feed may be produced with cervid protein (but not from positive CWD animals) in the United States (US), there is a greater than negligible risk that feed with cervid protein is imported from North America into GB. There is, however, uncertainty associated with this estimate.
In areas of North America where CWD has been reported, given that CWD is excreted in faeces, saliva, urine and blood, and survives in the environment for several years where it is able to bind to the soil, there is a medium probability that the environment (including soil) contains CWD.
Given the volume of tourists, hunters and servicemen moving between GB and North America, the probability of at least one person travelling to/from a CWD affected area and, in doing so, contaminating their clothing and/or equipment prior to arriving in GB is greater than negligible. For deer hunters, specifically, the risk is likely to be greater given the increased contact with deer and their environment. However, there is significant uncertainty associated with these estimates.
Once in GB, the use of animal feed is subject to the TSE Feed Ban and ABP Regulations. In accordance with the current ban, farmed deer should not be directly exposed to (i.e. feed) imported animal feed containing any PAP. Therefore, assuming this ban is strictly adhered to, the risk of farmed and wild deer being exposed to ruminant animal feed containing deer protein from North America is considered negligible but with associated uncertainty. The probability of a (wild) deer being exposed to CWD infected deer protein in non-ruminant feed is considered to be greater than negligible but uncertain.
The pathways by which naïve deer in GB may be exposed to CWD contaminated soil and prions on equipment and clothing from people arriving in GB from North America are variable and highly uncertain. Given associated uncertainty, there is a greater than negligible probability that a person could transfer CWD prions from their contaminated equipment and/or clothing into deer habitat/environment, particularly with respect to Roe deer (Capreolus capreolus) habitat but less so for Chinese Water deer (Hydropotes inermis) habitat. Further, given the volume of tourists and other travellers moving between North America and GB, there are potentially multiple opportunities for CWD prions to be transferred from equipment to the environment.
None of the species affected by CWD in North America are present in GB. For a British species to become infected with CWD given exposure will depend on the dose and inherent susceptibility. Based on current scientific evidence Red deer (Cervus elaphus elaphus) are susceptible to CWD, Fallow deer (Dama dama) are likely to be less susceptible and Roe deer (Capreolus capreolus) have a gene conferring susceptibility. Therefore, it is likely that given exposure to an infectious dose of CWD, deer in GB could become infected with CWD.
However, given that the amount of soil ingested is likely to be very small, the probability of ingesting an infectious dose via this route is considered negligible to very low. The probability of ingesting an infectious dose via consumption of nonruminant feed is likely to be higher and may be very low, with associated uncertainty.
Overall, the probability of importing CWD into GB from North America and causing infection in British deer is uncertain but likely to be negligible to very low via movement of deer hunters, other tourists and British servicemen and very low via imported (nonruminant) animal feed. However, if it was imported and (a) deer did become infected with CWD, the consequences would be severe as eradication of the disease is impossible, it is clinically indistinguishable from BSE infection in deer (Dagleish et al., 2008) and populations of wild and farmed deer would be under threat.
What is the risk of chronic wasting disease being introduced into Great Britain? A Qualitative Risk Assessment October 2012
Thursday, April 07, 2016
What is the risk of chronic wasting disease being introduced into Great Britain? An updated Qualitative Risk Assessment March 2016
Subject: DEFRA What is the risk of a cervid TSE being introduced from Norway into Great Britain? Qualitative Risk Assessment September 2016
Friday, September 30, 2016
DEFRA What is the risk of a cervid TSE being introduced from Norway into Great Britain? Qualitative Risk Assessment September 2016
Chronic wasting disease AND TISSUES THAT MIGHT CARRY A RISK FOR HUMAN FOOD AND ANIMAL FEED CHAINS REPORT
3.5.3 Conclusions
In deer and elk, PrPCWD has a very wide and early tissue distribution, which
resembles the distribution of scrapie and BSE agents in tissues in TSE-susceptible sheep and is different to that seen in BSE in cattle. However, tissue distribution is not identical for deer4 and elk. In the latter species it accumulates later in the incubation period into detectable levels. This widespread distribution of PrPCWD early in the incubation periodpresents significant, if not insurmountable, difficulty with respect to the potential for decisions on the removal of specified risk materials (SRM) in CWD.
FRIDAY, NOVEMBER 3, 2017
BSE MAD COW TSE PRION DISEASE PET FOOD FEED IN COMMERCE INDUSTRY VS TERRY S. SINGELTARY Sr. A REVIEW
''I have a neighbor who is a dairy farmer. He tells me that he knows of several farmers who feed their cattle expired dog food. These farmers are unaware of any dangers posed to their cattle from the pet food contents. For these farmers, the pet food is just another source of protein.''
IN CONFIDENCE
SATURDAY, NOVEMBER 4, 2017
FDA 589.2000, Section 21 C.F.R. Animal Proteins Prohibited in Ruminant Feed WARNING Letters and FEED MILL VIOLATIONS OBSERVATIONS 2017 to 2006
Thursday, November 16, 2017
Texas Natural Meats Recalls Beef Products Due To Possible Specified Risk Materials Contamination
CHRONIC WASTING DISEASE CWD TSE PRION ZOONOTIC ZOONOSIS
PRICE OF TSE PRION POKER GOES UP!
2017
Subject: ***CDC Now Recommends Strongly consider having the deer or elk tested for CWD before you eat the meat
CDC Now Recommends Strongly consider having the deer or elk tested for CWD before you eat the meat
Chronic Wasting Disease (CWD)
Prevention
If CWD could spread to people, it would most likely be through eating of infected deer and elk. In a 2006-2007 CDC survey of U.S. residents, nearly 20 percent of those surveyed said they had hunted deer or elk and more than two-thirds said they had eaten venison or elk meat. However, to date, no CWD infections have been reported in people.
Hunters must consider many factors when determining whether to eat meat from deer and elk harvested from areas with CWD, including the level of risk they are willing to accept. Hunters harvesting wild deer and elk from areas with reported CWD should check state wildlife and public health guidance to see whether testing of animals is recommended or required in a given state or region. In areas where CWD is known to be present, CDC recommends that hunters strongly consider having those animals tested before eating the meat.
Tests for CWD are monitoring tools that some state wildlife officials use to look at the rates of CWD in certain animal populations. Testing may not be available in every state, and states may use these tests in different ways. A negative test result does not guarantee that an individual animal is not infected with CWD, but it does make it considerably less likely and may reduce your risk of exposure to CWD.
To be as safe as possible and decrease their potential risk of exposure to CWD, hunters should take the following steps when hunting in areas with CWD:
Do not shoot, handle or eat meat from deer and elk that look sick or are acting strangely or are found dead (road-kill). When field-dressing a deer: Wear latex or rubber gloves when dressing the animal or handling the meat. Minimize how much you handle the organs of the animal, particularly the brain or spinal cord tissues. Do not use household knives or other kitchen utensils for field dressing. Check state wildlife and public health guidance to see whether testing of animals is recommended or required. Recommendations vary by state, but information about testing is available from many state wildlife agencies. Strongly consider having the deer or elk tested for CWD before you eat the meat. If you have your deer or elk commercially processed, consider asking that your animal be processed individually to avoid mixing meat from multiple animals. If your animal tests positive for CWD, do not eat meat from that animal. The U.S. Department of Agriculture’s Animal and Plant Health Inspection Service regulates commercially farmed deer and elk. The agency operates a national CWD herd certification program. As part of the voluntary program, states and individual herd owners agree to meet requirements meant to decrease the risk of CWD in their herds. Privately owned herds that do not participate in the herd certification program may be at increased risk for CWD.
Page last reviewed: August 17, 2017 Page last updated: August 17, 2017 Content source: Centers for Disease Control and Prevention National Center for Emerging and Zoonotic Infectious Diseases (NCEZID) Division of High-Consequence Pathogens and Pathology (DHCPP)
> However, to date, no CWD infections have been reported in people.
key word here is 'reported'. science has shown that CWD in humans will look like sporadic CJD. SO, how can one assume that CWD has not already transmitted to humans? they can't, and it's as simple as that. from all recorded science to date, CWD has already transmitted to humans, and it's being misdiagnosed as sporadic CJD. ...terry
LOOKING FOR CWD IN HUMANS AS nvCJD or as an ATYPICAL CJD, LOOKING IN ALL THE WRONG PLACES $$$
*** These results would seem to suggest that CWD does indeed have zoonotic potential, at least as judged by the compatibility of CWD prions and their human PrPC target. Furthermore, extrapolation from this simple in vitro assay suggests that if zoonotic CWD occurred, it would most likely effect those of the PRNP codon 129-MM genotype and that the PrPres type would be similar to that found in the most common subtype of sCJD (MM1).***
Molecular Barriers to Zoonotic Transmission of Prions
*** chronic wasting disease, there was no absolute barrier to conversion of the human prion protein.
*** Furthermore, the form of human PrPres produced in this in vitro assay when seeded with CWD, resembles that found in the most common human prion disease, namely sCJD of the MM1 subtype.
TUESDAY, SEPTEMBER 12, 2017
CDC Now Recommends Strongly consider having the deer or elk tested for CWD before you eat the meat
Prion 2017 Conference Abstracts CWD
2017 PRION CONFERENCE
First evidence of intracranial and peroral transmission of Chronic Wasting Disease (CWD) into Cynomolgus macaques: a work in progress
Stefanie Czub1, Walter Schulz-Schaeffer2, Christiane Stahl-Hennig3, Michael Beekes4, Hermann Schaetzl5 and Dirk Motzkus6 1
University of Calgary Faculty of Veterinary Medicine/Canadian Food Inspection Agency; 2Universitatsklinikum des Saarlandes und Medizinische Fakultat der Universitat des Saarlandes; 3 Deutsches Primaten Zentrum/Goettingen; 4 Robert-Koch-Institut Berlin; 5 University of Calgary Faculty of Veterinary Medicine; 6 presently: Boehringer Ingelheim Veterinary Research Center; previously: Deutsches Primaten Zentrum/Goettingen
This is a progress report of a project which started in 2009. 21 cynomolgus macaques were challenged with characterized CWD material from white-tailed deer (WTD) or elk by intracerebral (ic), oral, and skin exposure routes. Additional blood transfusion experiments are supposed to assess the CWD contamination risk of human blood product. Challenge materials originated from symptomatic cervids for ic, skin scarification and partially per oral routes (WTD brain). Challenge material for feeding of muscle derived from preclinical WTD and from preclinical macaques for blood transfusion experiments. We have confirmed that the CWD challenge material contained at least two different CWD agents (brain material) as well as CWD prions in muscle-associated nerves.
Here we present first data on a group of animals either challenged ic with steel wires or per orally and sacrificed with incubation times ranging from 4.5 to 6.9 years at postmortem. Three animals displayed signs of mild clinical disease, including anxiety, apathy, ataxia and/or tremor. In four animals wasting was observed, two of those had confirmed diabetes. All animals have variable signs of prion neuropathology in spinal cords and brains and by supersensitive IHC, reaction was detected in spinal cord segments of all animals. Protein misfolding cyclic amplification (PMCA), real-time quaking-induced conversion (RT-QuiC) and PET-blot assays to further substantiate these findings are on the way, as well as bioassays in bank voles and transgenic mice.
At present, a total of 10 animals are sacrificed and read-outs are ongoing. Preclinical incubation of the remaining macaques covers a range from 6.4 to 7.10 years. Based on the species barrier and an incubation time of > 5 years for BSE in macaques and about 10 years for scrapie in macaques, we expected an onset of clinical disease beyond 6 years post inoculation.
PRION 2017 DECIPHERING NEURODEGENERATIVE DISORDERS
Subject: PRION 2017 CONFERENCE DECIPHERING NEURODEGENERATIVE DISORDERS VIDEO
PRION 2017 CONFERENCE DECIPHERING NEURODEGENERATIVE DISORDERS
*** PRION 2017 CONFERENCE VIDEO
TUESDAY, JUNE 13, 2017
PRION 2017 CONFERENCE ABSTRACT
First evidence of intracranial and peroral transmission of Chronic Wasting Disease (CWD) into Cynomolgus macaques: a work in progress
TUESDAY, JULY 04, 2017
*** PRION 2017 CONFERENCE ABSTRACTS ON CHRONIC WASTING DISEASE CWD TSE PRION ***
TUESDAY, JUNE 13, 2017
PRION 2017 CONFERENCE ABSTRACT Chronic Wasting Disease in European moose is associated with PrPSc features different from North American CWD
Wednesday, May 24, 2017
PRION2017 CONFERENCE VIDEO UPDATE 23 – 26 May 2017 Edinburgh UPDATE 1
SATURDAY, JULY 29, 2017
Risk Advisory Opinion: Potential Human Health Risks from Chronic Wasting Disease CFIA, PHAC, HC (HPFB and FNIHB), INAC, Parks Canada, ECCC and AAFC
Prion Infectivity in Fat of Deer with Chronic Wasting Disease▿
Brent Race#, Kimberly Meade-White#, Richard Race and Bruce Chesebro* + Author Affiliations
In mice, prion infectivity was recently detected in fat. Since ruminant fat is consumed by humans and fed to animals, we determined infectivity titers in fat from two CWD-infected deer. Deer fat devoid of muscle contained low levels of CWD infectivity and might be a risk factor for prion infection of other species.
Prions in Skeletal Muscles of Deer with Chronic Wasting Disease
Here bioassays in transgenic mice expressing cervid prion protein revealed the presence of infectious prions in skeletal muscles of CWD-infected deer, demonstrating that humans consuming or handling meat from CWD-infected deer are at risk to prion exposure.
*** WDA 2016 NEW YORK ***
We found that CWD adapts to a new host more readily than BSE and that human PrP was unexpectedly prone to misfolding by CWD prions. In addition, we investigated the role of specific regions of the bovine, deer and human PrP protein in resistance to conversion by prions from another species. We have concluded that the human protein has a region that confers unusual susceptibility to conversion by CWD prions.
Wildlife Disease Risk Communication Research Contributes to Wildlife Trust Administration Exploring perceptions about chronic wasting disease risks among wildlife and agriculture professionals and stakeholders
Zoonotic Potential of CWD Prions: An Update Prion 2016 Tokyo Zoonotic Potential of CWD Prions: An Update
Ignazio Cali1, Liuting Qing1, Jue Yuan1, Shenghai Huang2, Diane Kofskey1,3, Nicholas Maurer1, Debbie McKenzie4, Jiri Safar1,3,5, Wenquan Zou1,3,5,6, Pierluigi Gambetti1, Qingzhong Kong1,5,6
1Department of Pathology, 3National Prion Disease Pathology Surveillance Center, 5Department of Neurology, 6National Center for Regenerative Medicine, Case Western Reserve University, Cleveland, OH 44106, USA.
4Department of Biological Sciences and Center for Prions and Protein Folding Diseases, University of Alberta, Edmonton, Alberta, Canada,
2Encore Health Resources, 1331 Lamar St, Houston, TX 77010
Chronic wasting disease (CWD) is a widespread and highly transmissible prion disease in free-ranging and captive cervid species in North America. The zoonotic potential of CWD prions is a serious public health concern, but the susceptibility of human CNS and peripheral organs to CWD prions remains largely unresolved. We reported earlier that peripheral and CNS infections were detected in transgenic mice expressing human PrP129M or PrP129V. Here we will present an update on this project, including evidence for strain dependence and influence of cervid PrP polymorphisms on CWD zoonosis as well as the characteristics of experimental human CWD prions.
PRION 2016 TOKYO
In Conjunction with Asia Pacific Prion Symposium 2016
PRION 2016 Tokyo
Prion 2016
Cervid to human prion transmission
Kong, Qingzhong
Case Western Reserve University, Cleveland, OH, United States
Abstract
Prion disease is transmissible and invariably fatal. Chronic wasting disease (CWD) is the prion disease affecting deer, elk and moose, and it is a widespread and expanding epidemic affecting 22 US States and 2 Canadian provinces so far. CWD poses the most serious zoonotic prion transmission risks in North America because of huge venison consumption (>6 million deer/elk hunted and consumed annually in the USA alone), significant prion infectivity in muscles and other tissues/fluids from CWD-affected cervids, and usually high levels of individual exposure to CWD resulting from consumption of the affected animal among often just family and friends. However, we still do not know whether CWD prions can infect humans in the brain or peripheral tissues or whether clinical/asymptomatic CWD zoonosis has already occurred, and we have no essays to reliably detect CWD infection in humans. We hypothesize that:
(1) The classic CWD prion strain can infect humans at low levels in the brain and peripheral lymphoid tissues;
(2) The cervid-to-human transmission barrier is dependent on the cervid prion strain and influenced by the host (human) prion protein (PrP) primary sequence;
(3) Reliable essays can be established to detect CWD infection in humans;and
(4) CWD transmission to humans has already occurred. We will test these hypotheses in 4 Aims using transgenic (Tg) mouse models and complementary in vitro approaches.
Aim 1 will prove that the classical CWD strain may infect humans in brain or peripheral lymphoid tissues at low levels by conducting systemic bioassays in a set of "humanized" Tg mouse lines expressing common human PrP variants using a number of CWD isolates at varying doses and routes. Experimental "human CWD" samples will also be generated for Aim 3.
Aim 2 will test the hypothesis that the cervid-to-human prion transmission barrier is dependent on prion strain and influenced by the host (human) PrP sequence by examining and comparing the transmission efficiency and phenotypes of several atypical/unusual CWD isolates/strains as well as a few prion strains from other species that have adapted to cervid PrP sequence, utilizing the same panel of humanized Tg mouse lines as in Aim 1.
Aim 3 will establish reliable essays for detection and surveillance of CWD infection in humans by examining in details the clinical, pathological, biochemical and in vitro seeding properties of existing and future experimental "human CWD" samples generated from Aims 1-2 and compare them with those of common sporadic human Creutzfeldt-Jakob disease (sCJD) prions.
Aim 4 will attempt to detect clinical CWD-affected human cases by examining a significant number of brain samples from prion-affected human subjects in the USA and Canada who have consumed venison from CWD-endemic areas utilizing the criteria and essays established in Aim 3. The findings from this proposal will greatly advance our understandings on the potential and characteristics of cervid prion transmission in humans, establish reliable essays for CWD zoonosis and potentially discover the first case(s) of CWD infection in humans.
Public Health Relevance There are significant and increasing human exposure to cervid prions because chronic wasting disease (CWD, a widespread and highly infectious prion disease among deer and elk in North America) continues spreading and consumption of venison remains popular, but our understanding on cervid-to-human prion transmission is still very limited, raising public health concerns. This proposal aims to define the zoonotic risks of cervid prions and set up and apply essays to detect CWD zoonosis using mouse models and in vitro methods. The findings will greatly expand our knowledge on the potentials and characteristics of cervid prion transmission in humans, establish reliable essays for such infections and may discover the first case(s) of CWD infection in humans.
Funding Agency Agency National Institute of Health (NIH)
Institute National Institute of Neurological Disorders and Stroke (NINDS)
Type Research Project (R01)
Project # 1R01NS088604-01A1
Application # 9037884
Study Section Cellular and Molecular Biology of Neurodegeneration Study Section (CMND)
Program Officer Wong, May
Project Start 2015-09-30
Project End 2019-07-31
Budget Start 2015-09-30
Budget End 2016-07-31
Support Year 1
Fiscal Year 2015
Total Cost $337,507
Indirect Cost $118,756
Institution
Name Case Western Reserve University
Department Pathology
Type Schools of Medicine
DUNS # 077758407
City Cleveland
State OH
Country United States
Zip Code 44106
***check out this old document. some interesting stuff about USA and other Countries and concern for CWD TSE Prion. ...terry
P169 Low levels of classical BSE infectivity in rendered fat tissue
Dr. Christine Fast1, Dr. Markus Keller2, Dr. Ute Ziegler3, Prof. Dr. Martin Groschup4 1Friedrich-Loeffler-Institut, Greifswald, Germany, 2Friedrich-Loeffler-Institut, Greifswald, Germany, 3Friedrich-Loeffler-Institut, Greifswald, Germany, 4Friedrich-Loeffler-Institut, Greifswald, Germany
Aims: Specified Risk Materials (SRM) are the animal tissues potentially containing the highest levels of Bovine Spongiform Encephalopathy (BSE) prions; and their removal is the most important consumer protection measure against BSE. BSE infectivity in the mesentery fat is most likely associated with embedded nervous tissue. To date, it is unclear if contamination of the rendered fat could have occurred during tallow production at a slaughterhouse.
Methods: Samples were taken from five cattle originating from the German BSE pathogenesis study. Two animals were at preclinical, one at late preclinical and one animal at clinical stage of disease; one control animal was included. For all cattle, mouse bioassay results for the celiac and mesenteric ganglion complex (CMGC) were generated previously, showing either no, mild, moderate or substantial infectivity loads. Fat was rendered from CMGC samples embedded in mesentery fat by incubating for 20 minutes at 95°C, according to standard tallow production methods. Subsequently, the melted fat was 1:5 diluted in physiological saline and thoroughly vortexed. The liquid fat was cleaned by a short centrifugation at 10.000 rpm. Finally, 7-12 bovine prion protein overexpressing transgenic mice (Tgbov XV) were i.c. inoculated with 25-30 μl of the supernatant. Mice were sacrificed after 730 days or when showing clinical symptoms and mouse brains were subsequently examined by biochemical and immunohistochemical methods.
Results: Neither the control and the preclinical nor the late preclinical animals showed signs of infectivity in mouse bioassay of the fat samples after up to 730 days p.i. In contrast, low levels of infectivity were detected in the fat of the clinical animal as one mouse displayed a clear accumulation of pathological prion protein in the brain after an incubation period of 598 days p.i.
Conclusions: Our results clearly indicate the potential contamination of melted mesenteric fat by embedded nervous structures during standard tallow production. However, the BSE infectivity level was weak and detectable only in the fat rendered from one sample with documented high infectivity load in the ganglion itself (Kaatz et al. 2012). Albeit, this study is not representative as only one clinical animal was included, it provides a proof of principle. A broader examination would allow a better insight into temporal and spatial distribution pattern of BSE infectivity in rendered fat tissues of different origins.Such estimates have a critical role in qualitative and quantitative risk assessments and in providing advice on the designation and removal of certain SRM tissues.
=====
http://prion2017.org/programme/
Prion Infectivity in Fat of Deer with Chronic Wasting Disease▿
Brent Race#, Kimberly Meade-White#, Richard Race and Bruce Chesebro* + Author Affiliations
Rocky Mountain Laboratories, 903 South 4th Street, Hamilton, Montana 59840 Next Section ABSTRACT
Chronic wasting disease (CWD) is a neurodegenerative prion disease of cervids. Some animal prion diseases, such as bovine spongiform encephalopathy, can infect humans; however, human susceptibility to CWD is unknown. In ruminants, prion infectivity is found in central nervous system and lymphoid tissues, with smaller amounts in intestine and muscle. In mice, prion infectivity was recently detected in fat. Since ruminant fat is consumed by humans and fed to animals, we determined infectivity titers in fat from two CWD-infected deer. Deer fat devoid of muscle contained low levels of CWD infectivity and might be a risk factor for prion infection of other species.
http://jvi.asm.org/content/83/18/9608.full
Prions in Skeletal Muscles of Deer with Chronic Wasting Disease
Rachel C. Angers1,*, Shawn R. Browning1,*,†, Tanya S. Seward2, Christina J. Sigurdson4,‡, Michael W. Miller5, Edward A. Hoover4, Glenn C. Telling1,2,3,§ ↵* These authors contributed equally to this work. ↵† Present address: Department of Infectology, Scripps Research Institute, 5353 Parkside Drive, RF-2, Jupiter, FL 33458, USA. ↵‡ Present address: Institute of Neuropathology, University of Zurich, Schmelzbergstrasse 12, 8091 Zurich, Switzerland. + See all authors and affiliations Science 24 Feb 2006: Vol. 311, Issue 5764, pp. 1117 DOI: 10.1126/science.1122864 Article Figures & Data Info & Metrics eLetters PDF You are currently viewing the abstract.
View Full Text
Abstract
The emergence of chronic wasting disease (CWD) in deer and elk in an increasingly wide geographic area, as well as the interspecies transmission of bovine spongiform encephalopathy to humans in the form of variant Creutzfeldt Jakob disease, have raised concerns about the zoonotic potential of CWD. Because meat consumption is the most likely means of exposure, it is important to determine whether skeletal muscle of diseased cervids contains prion infectivity. Here bioassays in transgenic mice expressing cervid prion protein revealed the presence of infectious prions in skeletal muscles of CWD-infected deer, demonstrating that humans consuming or handling meat from CWD-infected deer are at risk to prion exposure.
http://science.sciencemag.org/content/311/5764/1117.long
***Moreover, sporadic disease has never been observed in breeding colonies or primate research laboratories, most notably among hundreds of animals over several decades of study at the National Institutes of Health25, and in nearly twenty older animals continuously housed in our own facility.***
O.05: Transmission of prions to primates after extended silent incubation periods: Implications for BSE and scrapie risk assessment in human populations
Emmanuel Comoy, Jacqueline Mikol, Valerie Durand, Sophie Luccantoni, Evelyne Correia, Nathalie Lescoutra, Capucine Dehen, and Jean-Philippe Deslys Atomic Energy Commission; Fontenay-aux-Roses, France
Prion diseases (PD) are the unique neurodegenerative proteinopathies reputed to be transmissible under field conditions since decades. The transmission of Bovine Spongiform Encephalopathy (BSE) to humans evidenced that an animal PD might be zoonotic under appropriate conditions. Contrarily, in the absence of obvious (epidemiological or experimental) elements supporting a transmission or genetic predispositions, PD, like the other proteinopathies, are reputed to occur spontaneously (atpical animal prion strains, sporadic CJD summing 80% of human prion cases). Non-human primate models provided the first evidences supporting the transmissibiity of human prion strains and the zoonotic potential of BSE. Among them, cynomolgus macaques brought major information for BSE risk assessment for human health (Chen, 2014), according to their phylogenetic proximity to humans and extended lifetime. We used this model to assess the zoonotic potential of other animal PD from bovine, ovine and cervid origins even after very long silent incubation periods.
*** We recently observed the direct transmission of a natural classical scrapie isolate to macaque after a 10-year silent incubation period,
***with features similar to some reported for human cases of sporadic CJD, albeit requiring fourfold long incubation than BSE. Scrapie, as recently evoked in humanized mice (Cassard, 2014),
***is the third potentially zoonotic PD (with BSE and L-type BSE),
***thus questioning the origin of human sporadic cases.
We will present an updated panorama of our different transmission studies and discuss the implications of such extended incubation periods on risk assessment of animal PD for human health.
===============
***thus questioning the origin of human sporadic cases***
===============
***our findings suggest that possible transmission risk of H-type BSE to sheep and human. Bioassay will be required to determine whether the PMCA products are infectious to these animals.
==============
Transmission data also revealed that several scrapie prions propagate in HuPrP-Tg mice with efficiency comparable to that of cattle BSE. While the efficiency of transmission at primary passage was low, subsequent passages resulted in a highly virulent prion disease in both Met129 and Val129 mice. Transmission of the different scrapie isolates in these mice leads to the emergence of prion strain phenotypes that showed similar characteristics to those displayed by MM1 or VV2 sCJD prion. These results demonstrate that scrapie prions have a zoonotic potential and raise new questions about the possible link between animal and human prions.
Saturday, April 23, 2016
Scrapie ZOONOSIS PRION CONFERENCE TOKYO 2016
*** SCRAPIE WS-01: Prion diseases in animals and zoonotic potential 2016 ***
Prion. 10:S15-S21. 2016 ISSN: 1933-6896 printl 1933-690X
Volume 2: Science
4. The link between BSE and vCJD
4.29 The evidence discussed above that vCJD is caused by BSE seems overwhelming. Uncertainties exist about the cause of CJD in farmers, their wives and in several abattoir workers. It seems that farmers at least might be at higher risk than others in the general population. 1 Increased ascertainment (ie, increased identification of cases as a result of greater awareness of the condition) seems unlikely, as other groups exposed to risk, such as butchers and veterinarians, do not appear to have been affected. The CJD in farmers seems to be similar to other sporadic CJD in age of onset, in respect to glycosylation patterns, and in strain-typing in experimental mice. Some farmers are heterozygous for the methionine/valine variant at codon 129, and their lymphoreticular system (LRS) does not contain the high levels of PrPSc found in vCJD. It remains a remote possibility that when older people contract CJD from BSE the resulting phenotype is like sporadic CJD and is distinct from the vCJD phenotype in younger people.
4.30 Estimates of the likely scale of a possible epidemic of vCJD are wide-ranging and the subject of much debate. To know the likely number of cases is very important, not least to enable preparations to be made for the care of victims, as well as to be able to draw up guidelines to reduce the risk of transmission from infected but asymptomatic people. Preliminary results of the study examining tonsil and appendix material for signs of infection were not informative in this regard and full results are awaited. A blood test that would allow the widespread screening of the population by a simple method is still being sought.
>>> MONDAY, NOVEMBER 06, 2017 <<<
*** Experimental transfusion of variant CJD-infected blood reveals previously uncharacterised prion disorder in mice and macaque ***
''On secondary and tertiary transmissions, however, the proportion of PrPres positive animals gradually increased to almost 100%. Recent communications suggest that a similar situation might exist in other models of experimental exposure to prions involving swine32 and cattle33. ''
''Experimental transfusion of variant CJD-infected blood reveals previously uncharacterised prion disorder in mice and macaque''
In conclusion, the range of incomplete syndromes that we observed between healthy carriers and typical vCJD indicates that multiple forms of prion variants can coexist and may emerge in different forms depending upon the conditions under which transmission occurred. This has obvious consequences for public health, and questions the uniqueness of the BSE/vCJD strain50 and our capacity to detect and prevent all infectious forms of prion disease.
SATURDAY, DECEMBER 02, 2017
Public health risks from subclinical variant CJD
2001 FDA CJD TSE Prion Singeltary Submission
*** U.S.A. 50 STATE BSE MAD COW CONFERENCE CALL Jan. 9, 2001
DECEMBER 14, 2017, 20 YEARS POST DOD MOM HEIDENHAIN VARIANT CREUTZFELDT JAKOB DISEASE HVCJD DECEMBER 14, 1997, JUST MADE A PROMISE TO MOM, AND YOU DON'T BREAK PROMISES WITH YOUR MOM, NEVER FORGET, AND NEVER LET THEM FORGET...
wasted days and wasted nights...Freddy Fender
TERRY S. SINGELTARY SR.
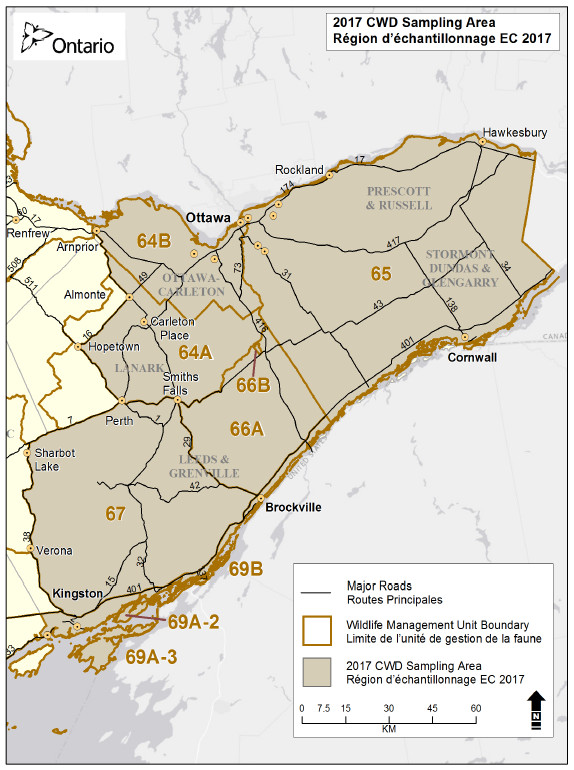


0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home